Titanium alloy is widely used in the biomedical field due to its excellent mechanical properties and biocompatibility. But titanium alloy and titanium have biological inertia, mainly through physical chimerism in the body, which is easy to cause loosening and falling off in the long-term use. In addition, due to the difference in thermal expansion coefficient between titanium and bone, the instability of the bond is also caused. Therefore, different titanium surface modification techniques have been proposed to meet the needs of clinical application. Common surface modification methods of implant materials mainly include: loose and rough surface treatment of implant materials; The surface coating of the implanted material is loaded with bioactive molecules or materials for surface modification.
Studies have shown that the main factors affecting the biocompatibility and osteointegration ability of implant materials mainly include material surface wettability, roughness, composition and crystal type. In the humoral environment, good surface wettability of implant material is more conducive to protein adsorption and cell adhesion. In addition, specific surface conditions are more conducive to cell differentiation and growth. Through the surface modification process of titanium alloy, a suitably modified coating can be constructed on the alloy surface to optimize the surface structure, composition and wettability of the alloy while maintaining the corrosion resistance and mechanical properties of the alloy, so as to achieve the improvement of compatibility and bone integration ability.
At present, the common surface modification coatings of medical titanium alloy for bone implantation mainly include hydroxyapatite (HA) coating, Graphene coating, chitosan coating and TiO2 nanotube array coating, among which, TiO2 nanotube array can be combined with other coatings to achieve better functions due to its in situ self-growing porous structure coating. In the future, the main direction of medical titanium alloy surface modification is to improve the bone integration ability of implants by preparing surface-modified coatings and combining various surface modification methods.
Hydroxyapatite (HA) Coating
As the main inorganic component of human bone, hydroxyapatite (HA) has good biocompatibility. Upon contact with body fluids, HA surface ions can be exchanged with ions in an aqueous solution, and molecules such as collagen and proteins or ions are adsorbed on their surfaces to produce biofilms and coatings. HA coating not only combines chemical bonds on the bone/implant closely but also as a barrier between the fluid and metal implants, which depends on its preparation methods and technology, influencing the bonding strength, crystallinity and density matrix, the combination of low intensity can lead to modification of failure, Flaking of the HA coating can cause inflammation and other problems. The results show that the adhesion strength of HA coating by magnetron sputtering can reach 80 MPa, which is higher than the adhesion strength of coating prepared by hot isostatic pressing, pulse laser deposition, plasma spraying and sol-gel method (about 14MPa, 16MPa, 25MPa and 26 MPa respectively).
At present, plasma spraying and electrophoretic deposition are commonly used to prepare HA coating, the latter can be used for complex matrix coating. The HA coating prepared by thermal spraying was annealed to reduce the residual stress, and the bonding strength of the HA coating increased obviously because the residual stress was reduced by heat treatment. In order to improve the adhesion strength of HA coating, the surface of titanium alloy can be roughened by various pretreatment techniques such as electron beam etching, microsphere blasting, acidizing etching and sandpaper grinding, or the transition layer can be deposited between HA coating and titanium alloy substrate.
The crystallinity of HA coating affects cell behavior. Compared with high crystallinity HA coating, low crystallinity HA coating shows a lower proliferation rate of osteoblasts. It was found that the HA nano-coating and micron coating with different crystallinity showed different dissolution and reprecipitation characteristics, and the inqualitative HA showed high solubility in vivo. It is speculated that the early bone formation kinetics is related to the solubility of HA coating. Controlled crystallization of HA coating can be realized by annealing or deposition at high temperatures (700~800 ℃). Part of amorphous coating is transformed into crystalline coating during the annealing process and HA coating with certain crystallinity or ion-substituted structure can be obtained. Compared with the sheet HA coating, the acicular structure coating is dense and uniform, providing more contact area with the surrounding fluid and therefore more suitable for apatite deposition. The microstructure of HA coating can also be changed by heating and sintering. Hulbert et al. showed that the porous structure requires oxide ceramics with a minimum interconnecting pore size of about 100 μm for new bone tissue to grow inward and provide space for fluid circulation. They found that the smaller pore size allows incomplete mineralization of the permeable tissue. Completely dense HA coating is not conducive to cell proliferation and differentiation, mainly used as bone formation scaffold, resulting in its limited ability to induce bone formation.
Graphene Coating
In 2004, the British physicist at the University of Manchester Geim and Novoselov graphite by tape micro mechanical separation successfully isolated monolayer of carbon atoms in the structure, namely the points of graphene make stone material with high specific surface area, high conductive thermal conductivity, low density, excellent physical properties, the two scientists won the Nobel Prize for physics in 2010. The scientists found that small molecules by different groups of chemical modification can form different graphene derivatives, such as graphene oxide, reduction of graphene oxide, carbon nanotubes, etc., these materials with different properties of the graphene material family, often used for biological material modification is oxidized graphene derivatives. In a large number of studies on graphene and its derivatives modified composite materials promoting osteogenesis, researchers found that graphene loaded scaffold materials showed better cytocompatibility and osteoregeneration induction ability, and explored the mechanism of its promoting bone regeneration. Kumar et al. demonstrated that go increased the uptake of osteogenic factors in human mesenchymal stem cells in vitro, thereby promoting osteogenic differentiation of stem cells. Graphene has been shown to concentrate dexamethasone and 0-glycerophosphate, two classic osteogenic inducers, in culture to promote the differentiation of human bone marrow mesenchymal stem cells into osteoblasts. In addition, rGO light apatite composite can regulate the expression of bone-related proteins and promote matrix maturation and calcification.
Chitosan Coating
As a natural organic compound, chitosan has the advantages of good biocompatibility, non-toxicity, good antibacterial and promoting cell proliferation and differentiation, and is often used as surface modification coating for titanium alloy implants. Chitosan also has good adsorption capacity, can be biodegradable in vivo, and can be combined with hydroxyapatite and other substances as a carrier. The thickness of chitosan coating can be controlled to control the local concentration of drugs to treat postoperative infection and inflammation, so that the implant can achieve better bone integration and rapid healing. Chitosan can interact with negatively charged bacterial cells to achieve the antibacterial effect, but it is slightly less antibacterial than metal ions, which reduces the risk of implantation failure.
TiO2 Nanotube Array coating
TiO2 nanotube structure can prevent the release of metal ions (such as Al, V, etc.) and alleviate the implantation reaction, showing better corrosion resistance and biocompatibility than TiO2 bulk materials. Therefore, the synthesis of TiO2 nanotube array coating on titanium alloy surface becomes an effective measure to improve its medical performance.
Anodic oxidation is often used for TiO2 nanotube arrays on the surface of titanium materials, so as to form TiO2 oxide layer coating with approximately skeletal porous structure. By changing anodic oxidation voltage and duration, the tube length and diameter of TiO2 nanotube arrays can be regulated. TiO2 nanotube arrays prepared by anodic oxidation were amorphous and could change from amorphous to anatase phase or rutile phase after annealing at 300-500 ℃, and gradually changed to rutile phase after annealing at 600 ℃. With the increase of surface crystallinity, the surface wettability of the nanotube array is improved, which makes protein adsorption and cell adhesion easier. The synergistic effect of the nanotube and crystal structure accelerates the deposition of hydroxyapatite. The results show that anatase phase is superior to rutile phase in inducing cell differentiation or cell proliferation and the former phase is more likely to deposit hydroxyapatite. When the anodic oxidation voltage is within a certain range, the length and diameter of the nanotubes increase with the increase of the voltage. As the oxidation time increases, the surface roughness increases and the contact angle decreases.
Ti-6Al-4V alloy is a relatively widely used biomedical material, which is composed of α+β phase. During anodic oxidation, the solubility of the two phases is different, and the length of nanotubes is also different in different phase regions. Mansoorianfar et al. successfully prepared TiO2 nanotube arrays with good uniformity on Ti-6Al-4V alloy by secondary anodic oxidation at a voltage of 50-75 V. The average tube length and diameter of nanotube increased with the increase of voltage(Showed in the figure below). The study found that the sample prepared at 60V voltage showed the best cell activity.
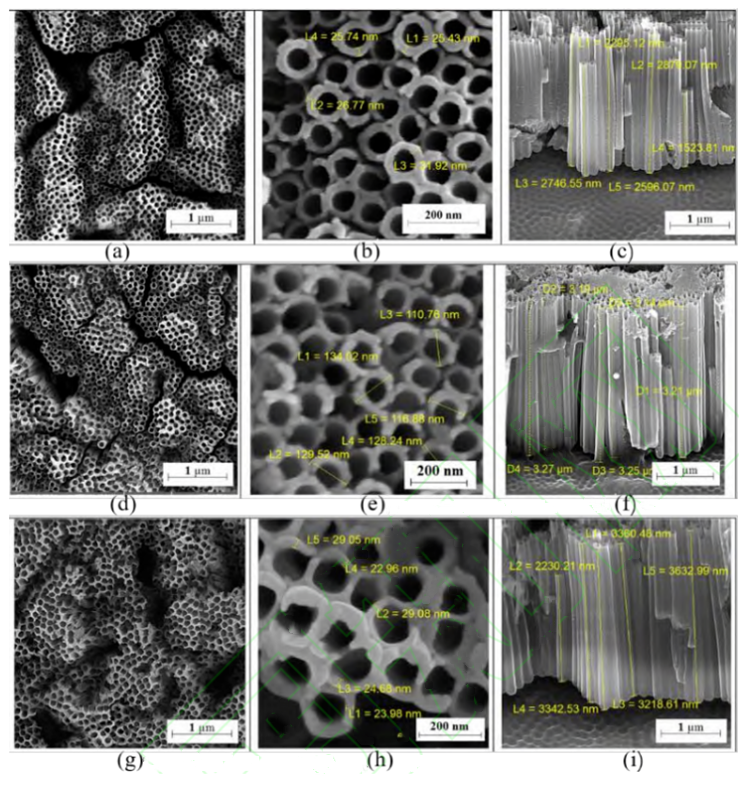
The preparation of TiO2 nanotube array layer on biotitanium alloy with low elastic modulus not only ensures the mechanical properties of the implant material but also improves the biocompatibility. Li et al. prepared TiO2 nanotube array layer on ti-24Nb-4Zr-7.9Sn (Ti2448) titanium alloy surface, and compared pure Ti, nanotube-Ti (NT) and Ti2448. Nanotube-ti2448 (NTi2448) showed higher wettability, corrosion resistance, cytocompatibility and bone integration ability. Due to the addition of Nb, Zr and other elements in titanium alloy with low elastic modulus, the oxidation film formed after anodic oxidation improves its corrosion resistance. In addition, the addition of alloying elements reduces the orderliness of nanotube array, and some studies have shown that arrays with low orderliness show better compatibility.
In The Last
Surface modification technology is a more effective way to improve the biological activity, wear resistance and antibacterial properties of titanium and titanium alloys, and to improve the existing conventional biomaterials to meet the current evolving clinical needs. Researchers have made a lot of attempts to improve the design and biocompatibility of new medical titanium alloys. The elastic modulus of the newly developed medical titanium alloys is increasingly close to the elastic modulus value of human bone tissue. The construction of modified coating on the surface of titanium alloys has greatly improved the biocompatibility, bone integration ability and antibacterial ability of the alloys. In addition, a variety of physical and chemical methods have been used to improve the wear performance of titanium alloy surfaces, by depositing a layer of ceramic coating with excellent wear resistance on the titanium surface, such as diamond-like carbon film (DLC), titanium nitride (TiN) coating.